Overview
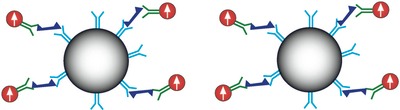
MagneSensors’ nanotechnology platform utilizes patented ultra high sensitivity superconducting quantum interference device (SQUID) sensors to detect the magnetic field generated by magnetic nanoparticles that are attached to biological molecules of interest. The targeted biological molecules are recognized by either antibodies or nucleic acid probes that we coat onto the magnetic nanoparticle labels.
Magnetic detection is entirely different from existing optical and radioactive platforms, overcoming some inherent limitations in other platforms and providing breakthrough advantages.
We have performed thousands of different proof-of-concept magnetic assays over the past decade. These include cell assays (both cell surface receptor and intracellular), sandwich immunoassays assays for proteins, and more recently, sandwich hybridization assays for the direct detection of nucleic acids (no amplification).
Advantages
Magnetic detection is entirely different from existing (e.g., optical) platforms, overcoming some inherent limitations and providing fundamental advantages. Particularly useful is the ability to perform ultra-sensitive mix and measure assays directly in complex matrices (e.g. blood, serum) since biological substances inherently do not generate a magnetic signal for our magnetic measurements. Our target applications benefit from a combination of these advantages that are not available with existing methods.
The key benefits are:
Major Benefits
· Ultra-high sensitivity, can quantitatively detect <1,000 labels (<attomoles for sandwich immunoassays, 40 cells for surface receptors)
· “Mix and measure” format enables fast results, easy to automate, limited sample handling as no need to separate out unbound labels
· Perform assays in serum and blood since minimal biological interference for magnetic detection
· Ability to apply forces to magnetic labels using magnetic gradient fields to reduce non specific binding and increase signal to noise
Other Benefits
· Inexpensive and non-toxic labels (iron oxide)
· Simple measurement, little training required
· Ability to reduce assay incubation time using magnetic fields to move ("stir") labels
· Capability for binding and real-time kinetics information
· High label stability, can re-measure samples, archive
Magnetic Assays
Basic Principles - Cell Assays
The basic principles of using magnetic detection with MNPs for immunoassays were first published by Kötitz in 1995. In magnetic assays, the MNP is the label. Our magnetic assays are all conveniently performed in microwells.
The figure below illustrates the principle of magnetic detection as applied to cell assays. Magnetic nanoparticles conjugated to detect antibodies against a cell surface antigen are added to microplate wells containing the target cells and incubated. To measure, the samples are briefly exposed (<1 sec) to a magnetic field applied with a small external magnet. This aligns all the dipole moments of the MNPs. The magnetic field is then removed and the samples are quickly moved (rotated) past the magnetic sensor that is located in very close proximity (in a manner roughly analogous to a hard disk drive).
A key point is after the external magnetic field is removed, the dipole moments of the unbound MNPs randomize very quickly (under 10ms for MNPs <300nm size) due to Brownian motion as the MNPs physically rotate in solution. Thus very soon after removal of the field, there is no net signal from the unbound MNPs.
MNPs bound to the cells have limited mobility (Brownian time constant >400 sec for 10 µm size cells) and their dipole moments remain aligned for relatively long periods of time, which is governed by Néel relaxation. After the field is removed, a net remanent magnetic signal can be measured proportional to the number of bound MNPs and thus the number of labeled cells. The high sensitivity stems from the use of our SQUID magnetic sensor, which can detect extremely small magnetic fields and hence can measure very small numbers of MNPs (1,000-2,500 MNPs).
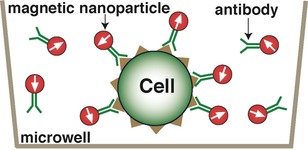
1. Add magnetic nanoparticle labels coated with detection antibody. Antibodies bind MNPs to surface antigen on target cell.
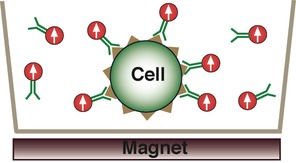
2. Apply magnetization field by moving sample over external magnet. All magnetic nanoparticle dipole moments align with the external magnetic field.
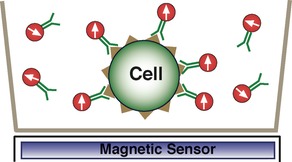
3. Remove sample from magnetic field, unbound labels quickly randomize, no net magnetic signal. Measure magnetic signal only from bound labels as all bound labels remain aligned with field.
Note cells are large and remain stationary over time window of measurement.
Basic Principles - Sandwich Immunoassays
The basic principle for a sandwich immunoassay is similar to those described above for the cell assay. Non magnetic microspheres are used to capture the target antigen in solution (improving kinetics compared to capturing on the microwell surface).
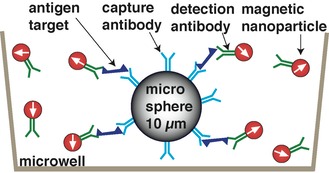
1. Bind target antigen to microsphere coated with capture antibody. Sandwich antigen with magnetic nanoparticle labels conjugated to detection antibody.
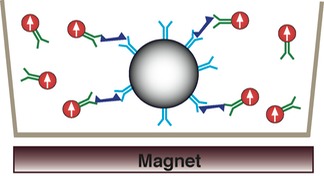
2. Apply magnetization field by moving sample over external magnet. All magnetic nanoparticle dipole moments align with the external magnetic field.
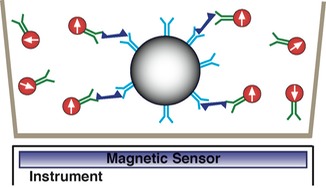
3. Remove sample from magnetic field, unbound labels quickly randomize, no net magnetic signal. Measure magnetic signal only from bound labels as all bound labels remain aligned with field.
Note that non magentic microspheres are large and remain stationary over time window of measurement.
Core Nanotechnologies
Our core competencies consist of two key nanomagnetic technologies.
Magnetic Sensors
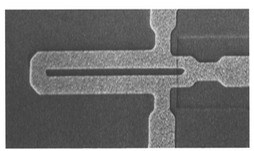
The most critical core technology is the fabrication of ultra-sensitive magnetic sensors from high temperature superconductors. Superconducting Quantum Interference Devices (SQUIDs) are the most sensitive class of magnetic field detectors known. The SQUID sensor can be most simply thought of as an ultra sensitive amplifier. The SQUID magnetometer incorporates an integrated pickup coil, which can be viewed analogous to an antenna.
The SQUID magnetic sensor forms the crucial component in our new instrumentation designed to detect small (50-500 nm) magnetic nanoparticles. Using high temperature superconductors (which garnered the Nobel prize in Physics in 1987), SQUIDs can be operated simply and inexpensively using a small refrigerator called a cryocooler. With a refrigerator, the cooling that used to be a significant obstacle to bench top instrumentation, is now essentially invisible to the user.
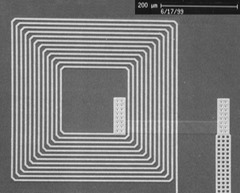
Historically, the challenge has been in developing high temperature SQUIDs that are sensitive, reliable, and manufacturable in quantity. We developed and patented a key junction nanotechnology that readily enables the fabrication of very sensitive high temperature SQUIDs that are robust (SQUID chips in our lab often last over five years and have been used to measure over a thousand magnetic assays). The junction fabrication relies upon the ability to easily and reproducibly fabricate junctions with very small (10-40 nm) dimensions. The unique combination of MagneSensors device sensitivity and manufacturability was first published in Nature. Along with reliability, these set MagneSensors apart from potential competitors for commercialization of the technology.
The SQUID sensors are fabricated in a clean room environment using equipment and processes that have been adapted from the semiconductor industry. These techniques consist of depositing and patterning thin films of superconductors on wafers.
Magnetic Nanoparticles
The innovation centers on the synthesis of next generation magnetic nanoparticle labels that are tailored specifically for magnetic detection. Most commercially developed magnetic nanoparticles are made for cell separation and sorting applications and are not well suited for magnetic detection (aggregation, too large, too weak, poor coating).
Our magnetic nanoparticles are synthesized for: 1) larger magnetic moment and 2) surface chemistry modifications on the magnetic nanoparticles to attach antibodies (or DNA probes) and to enable stability in blood. Proprietary coatings are used to reduce non-specific binding and to minimize aggregation. We synthesize Fe3O4 magnetic nanoparticles for an optimal combination of high magnetic signal, ease of synthesis, and useful coatings.

Different magnetic detection assay applications also benefit from different size magnetic nanoparticles and our synthesis process allows us to produce optimally sized magnetic nanoparticles (currently 60-350 nm) for various applications.
We expect that these tailored magnetic nanoparticle labels will significantly increase the sensitivity of the magnetic assays and ultimately provide a sizable reagent revenue source.
Recent studies have also shown that our magnetic nanoparticles work very well for magnetic cell separation and hence could benefit this sizable and growing market.
Instrumentation
We have made major improvements to the magnetic detection instrumentation over the past decade. Our third generation instrument is designed for operation in a hospital environment. This bench top instrument utilizes our patented high temperature SQUID magnetic sensor and the cooling is entirely transparent to the user due to the incorporation of a small cryorefrigerator.
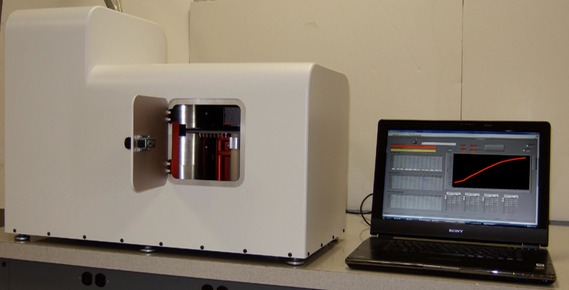
The innovative design allows the room temperature samples to be brought very close to the cooled high temperature SQUID sensor chip (operating below 77K), allowing for good magnetic coupling of the signal generated by the magnetic nanoparticles to the sensor. The magnetic sensor is also located inside small magnetic shields to prevent unwanted external magnetic noise sources from interfering with the sensitive measurements.
This magnetic assay instrument can measure 50 samples per minute, is easy to use, and requires very little training. The instrument design has similarities to a disk drive, where the samples are magnetized (similar to a “write head”) and rotated over the “read head”, namely the ultra-sensitive SQUID magnetic sensor chip. The samples are loaded and measured in removable strips of 8 microwells. Assays can be prepared in a 96 well format.

Photo of custom designed 8-well test strip that enables magnetic assays to be prepared in a 96 well format using 12 strips. The inexpensive multi-well test strip was specially designed for low magnetic background and loads into measurement system in seconds.
Intellectual Property
MagneSensors has broad patent coverage for its core technology. MagneSensors owns eight patents and has exclusive royalty-free licenses to five patents along with a non-exclusive royalty-free sublicense to key IBM technology (26 separate categories).
Perhaps even more importantly, MagneSensors has an extensive portfolio of highly developed proprietary fabrication processes as well as design and measurement techniques. MagneSensors’ core technology presents a very formidable barrier to entry, as it requires mastering complex superconducting materials systems using specialized processes, equipment, and highly trained personnel. MagneSensors is the only U.S. company, and one of only three or four companies worldwide, with such capability. Over the intervening years MagneSensors researchers have evolved the technology to the point where the sensors now have the requisite sensitivity and reliability for commercialization.
